The COVID-19 pandemic has profoundly affected many elements of life, and scientific research is no exception. Research related to SARS-CoV-2 and the pandemic is taking place at unprecedented speed and scale, and many scientists have seen their line of research refocused to support efforts in tackling the ongoing outbreak. At the same time, other scientists have seen their research activity affected by laboratory and institutional closures.
Research is being shared via more varied channels and much earlier than ever before. On the one hand, there is a moral imperative to share outputs as early as possible to support progress in tackling the outbreak. On the other hand, with labs and institutions closed and travel restrictions in place, there is a need for new modalities for research sharing and interacting with our communities.
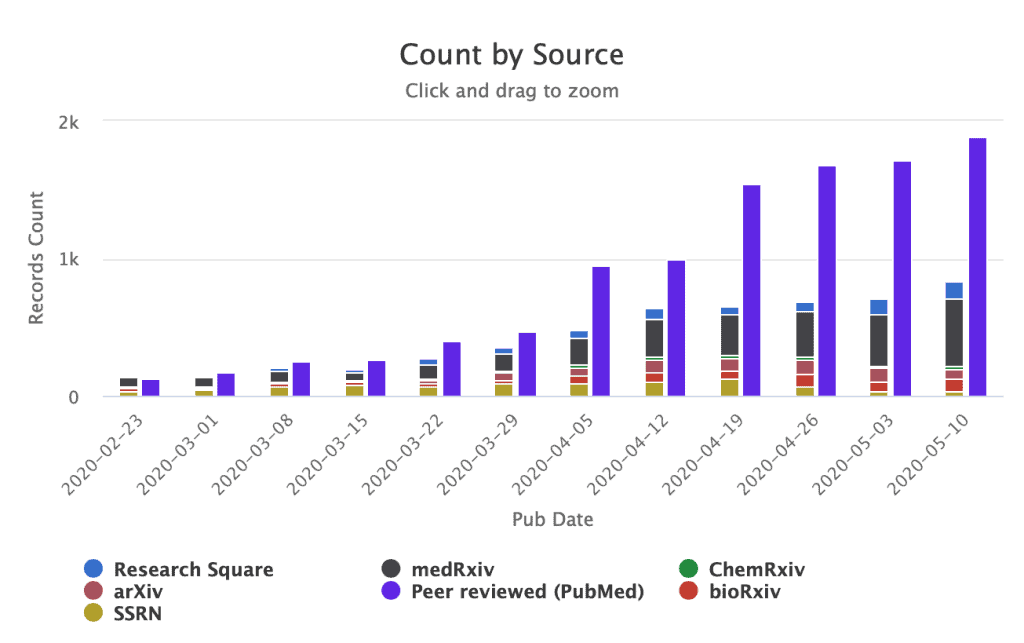
Preprints are one of the mechanisms that researchers are using to communicate their work early and broadly, and a significant fraction of COVID-19 research is currently available via preprints. As of 19 May 2020, 32% of the COVID-19 papers listed on the NIH’s OPA COVID-19 portfolio are preprints, by comparison, the proportion of biomedical preprints vs the published literature in PubMed stood at 3% in 2019 (source for 2019 here, current data here).
Preprints provide a mechanism for the rapid dissemination of research results, at the same time, given that preprints do not undergo peer review, concerns have been raised about potential risks to public health or misinformation to the public if research in preprints were to be used as established clinical evidence, and conversations have been sparked about how to find the balance between rapid dissemination of research and mitigating potential risks.
We include here a number of resources related to preprints for COVID-19, early communication of research during the pandemic, and initiatives towards openness that have arisen and are ongoing to support research progress in tackling this outbreak. If you have any items or resources you would like to see added, please contact Iratxe at iratxe.puebla@asapbio.org.
Contents
- Preprints for COVID-19 research
- What is a preprint
- Finding COVID-19 preprints
- The impact of COVID-19 preprints
- Review, commentary, and scrutiny on COVID-19 preprints
- Other mechanisms of rapid sharing of research outputs
- Sequence repositories
- Protocols and materials
- Other repositories
- Other rapid communication channels
- Journal policies
- In the News
Preprints for COVID-19 research
What is a preprint?
A preprint is a complete scientific manuscript that is uploaded by the authors to a public platform. The preprint is often the same or an earlier version of a manuscript submitted to a journal for peer review and publication.
After a brief quality-control inspection to ensure that the work is scientific in nature, the manuscript is posted on the preprint platform without peer review and can be viewed without charge by anyone in the world. Preprints allow scientists to directly control the dissemination of their work to the world-wide scientific community.
In most cases, the same work posted as preprint is also submitted for peer review at a journal. Preprints (rapid, but unvetted via journal peer-review) and journal publication (slower, but evaluated via peer review) can work in parallel as communication channels for scientific research. More information on preprints, their use in the life sciences and considerations around preprint posting is available via the ASAPbio Preprint FAQ.
Finding COVID-19 preprints
Preprints relevant to COVID-19 are being posted to a number of preprint platforms (a full directory of preprint platforms is available here). The preprint servers with large numbers of COVID-19 preprints are bioRxiv & medRxiv, arXiv, Research Square, Preprints.org, OSF, SSRN, and the WHO Bulletin.
A number of tools and repositories have been developed to surface COVID-19 research, many of which include preprints:
- The WHO COVID repository covers journal articles, and forms a subset of CORD-19 from the Allen Institute, which is updated weekly. It adds additional results from PubMed’s PMC open access corpus, as well as bioRxiv and medRxiv pre-prints.
- Lens’s COVID-19 collection covers scholarly works as well as patents.
- The NIH’s OPA COVID-19 portfolio covers content from PubMed and several preprint servers: arXiv, bioRxiv, medRxiv, ChemRxiv, Research Square, SSRN, Qeios, and preprints.org.
- The EBI-EMBL COVID-19 Data Portal facilitates data sharing and analysis around COVID-19 but also allows dedicated searches of literature around the coronavirus epidemic, including preprints.
- Dimensions has created an application listing all relevant publications, datasets and clinical trials related to Covid-19 available in the Dimensions dataset, this includes papers available as preprints.
- Several machine learning and AI-powered search and discovery tools have also created dashboards, including Google AI, Mendel, Ask Miso, Primer, and Meta.
- Europe PMC indexes preprints and provides a dedicated search for COVID-19 research: https://bit.ly/2CPRH6V
The impact of COVID-19 preprints
The PreLights team at Company of Biologists is curating relevant preprints at http://covidpreprints.com/.
Liam Brierly discusses ‘The role of research preprints in the academic response to the COVID-19 epidemic‘ and reviews their growth in comparison to previous infectious disease outbreaks.
In a piece in The Lancet Global Health, Majumder and Mandl discuss R0 estimates reported in preprints posted in January and in peer-reviewed studies published subsequently, and note that preprints are having an impact on discourse and decision making for the COVID-19 outbreak.
The preprint ‘Preprinting a pandemic: the role of preprints in the COVID-19 pandemic‘ reports an analysis of bioRxiv and medRxiv preprints about the COVID-19 pandemic (in the January 1 to April 30 period) , the results show that >40% of the total COVID-19 literature in that timeframe had been posted via preprints, and also that COVID-19 preprints are shorter and are being peer reviewed faster than non-COVID-19 preprints posted in the same time period.
Watch an ASAPbio/KFG webinar about the impact of sharing COVID-19 research via preprints and other rapid mechanisms.
Review, commentary, and scrutiny on COVID-19 preprints
NIH NIGMS and OASPA, a coalition of publishers, have called for more review and comments on preprints, with the latter writing that “The more rigorous and helpful review of preprints that can occur during this time, the better for all reviewers, authors, and editors.” Indeed, public commentary on preprints can help to contextualize research. For example, an infamous preprint was swiftly withdrawn after dozens of comments called attention to troubling flaws. Retraction Watch maintains a list of retracted/withdrawn COVID-19 papers, which includes preprints.
There are several organized communities working to provide feedback on COVID-19 preprints. For example, researchers at Precision Immunology Institute at the Icahn School of Medicine (PrIISM) operate the the Sinai Immunology Review Project, which has posted over 100 detailed reviews to bioRxiv and medRxiv. As described in this article, “workflow ranks each COVID-19-related preprint (totalling 1,772 to date) according to its immunological relevance. The most relevant papers are then reviewed in detail by trainees and validated by a faculty member.” Their dashboard displays rankings and review status of thousands of preprints.
Outbreak Science Rapid PREreview is a platform that facilitates rapid, open review of preprint related to outbreaks. Visitors can not only add their own reviews, but also request reviews of preprints. Reviews are highly structured, with quantitative scores that can be averaged across multiple reviews.

Publons hosts a portal listing COVID-19 related papers, including preprints, which allows readers to add reviews or scores to papers.
MITPress and the Berkeley School of Public Health have launched Rapid Reviews: COVID-19 (RR:C19), an open access, overlay journal that will select and rapidly review COVID-19 preprints.
Other mechanisms of rapid sharing of research outputs
Sequence repositories
Thousands of COVID-19 genomes have been submitted to the GISAID sequence repository. The data can be used to publish results if the publication acknowledges the originating laboratory and the authors agree to collaborate with the data provider in further analysis and research.” GISAID data also appears elsewhere on the web; for example via the Nexstrain visualization includes data from GISAID and provides downloadable metadata for attribution (github).
Protocols and materials
- Protosols.io has a dedicated section for methods and protocols related to coronavirus
- Research protocols and materials are also being shared on individual lab web pages, such as the ELISA methods shared by the Krammer lab and the Krogan lab.
- Addgene facilitates sharing of SARS-CoV-2 plasmids.
- The European virus archive distributes COVID-19 strains, detection kits and reagents.
Other repositories
General-purpose repositories also provide collections of COVID-19 research objects, such as the Figshare COVID-19 collection and the Zenodo COVID-19 community.
The Johns Hopkins 2019 Novel Coronavirus Visual Dashboard for epidemiological data makes its code and data available on github, and the COVID Tracking Project collates open data from multiple sources.
Other rapid communication channels
Researchers are using a variety of other channels to communicate rapidly. The Virological.org blog functions as a discussion forum, Imperial College reports are translated into multiple languages, and a Slack channel started by Dave O’Conner and Tom Friedrich, described in this article and also featured in an ASAPbio/KFG webinar on rapid communication of COVID-19 research.
Journal policies
In response to a statement coordinated by the Wellcome Trust, a group of publishers have committed to making relevant papers free to read for the duration of the pandemic.
A group of publishers and scholarly communications organizations (eLife, Hindawi, PeerJ, PLOS, Royal Society, F1000 Research, FAIRsharing, Outbreak Science, and PREreview) has launched an initiative to ensure a rapid, efficient, yet responsible review of COVID-19 content.
eLife has made changes to its policies on peer review in response to the impact of COVID-19 on the scientific community.
This preprint by Serge Horbach reports that the turnaround times for COVID-19 publications at medical journals have decreased on average by 49%, or 57 days, compared to publications in the same journal before the pandemic.
In the News
Research on COVID-19 has received wide coverage via media outlets. While some have raised concerns about the risk for misinformation in relation to non-peer reviewed work, the rapid and open sharing of research has also allowed errors to be addressed at a much faster pace than via traditional mechanisms. Journalists have also reported on how the need for rapid exchanges and collaboration to tackle the pandemic have resulted in increased adoption of open research practices.
- Nature News – ‘Will the pandemic permanently alter scientific publishing?‘
- BMJ feature- ‘Research on covid-19 is suffering “imperfect incentives at every stage”’
- The New York Times – ‘A Study Said Covid Wasn’t That Deadly. The Right Seized It.‘
- Nature News – ‘How swamped preprint servers are blocking bad coronavirus research‘
- The New York Times Magazine – ‘Coronavirus Is Forcing Medical Research to Speed Up‘
- The Economist – ‘Scientific research on the coronavirus is being released in a torrent‘
- Bloomberg Opinion – ‘A Pandemic Moves Peer Review to Twitter‘
- The Conversation – ‘Air pollution, COVID-19 and death: The perils of bypassing peer review‘
- Nautil.us – ‘Why False Claims About COVID-19 Refuse to Die‘
- Times Higher Education – ‘Huge Covid-19 output prompting ‘sea change’ in access to research‘
- Ars Technica – ‘The preprint problem: Unvetted science is fueling COVID-19 misinformation‘
